Millikan's oil drop experiment
This simulation is a simplified version of Robert Millikan's experiment. We change the electrical field to balance the gravitational force of the charged oil drops. The goal is to find the value of the charge of the electron.
Background
Robert Millikan wanted to find the value of the charge of the electron. During the years 1908-1910 he studied how small droplets of oil in an electrical field fall. By adjusting the strength of the electrical field he could balance the electrical force with the gravitational force.
Eventually, Millikan managed to calculate the charge of the electron. In 1923 he received the Nobel Prize in Physics for his achievement.
This experiment
The droplets in this simulation all have the same mass (m = 1.0·10-15 kg) but different negative electrical charge. If there is no electrical field all droplets will fall down due to gravity. When we increase the electrical field, we will find that some drops will start to "fall" upwards while some still fall downwards.
At certain field strengths some drops will float, this is when the electrical force Fe = q·E and the gravitational force Fg = m·g are equal, see figure 1. It is now possible to calculate the amount of electrical charge of a floating droplet.
Fe = Fg ⇒ qE = mg ⇒ q = mg/E
This tells us the total charge of the droplet, but we do not know how many electrons this corresponds to. By adjusting the strength of the electrical field it is possible to find different values of E that makes some other droplets float. When comparing these different values, we hopefully may find a pattern that will give us the charge of one electron.
This simple simulation focuses on the main principles of the experiment: the balance of electrical and gravitational forces and the quantization of electrical charge. In the real experiment not all drops have the same mass and we also have to consider that drops are affected by air resistance and a buoyant force from the surrounding air.
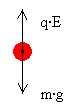
Figure 1 - Balance of forces
Figure 2 - The apparatus
Classroom activity
The students may preferably work in pairs. Each pair should have access to the web page with the simulation apparatus.
Using the slider, students should adjust the value of E until they find droplets that float. It can be difficult to determine whether a droplet is floating or just accelerate at a very slow rate. For each value where there seems to be floating droplets, the students should wait at least 10 seconds to be sure that the droplets are not moving up or down. When they have found a value of E that gives floating droplets they should write it down. When this is done, change the value of E to find differently charged droplets that float.
Instruct some pairs to start at the highest value of E and move down on the scale and some pairs to start from zero and move up. Some pairs might start in the middle and move down or up. In this way it is more probable that all interesting values of E will be found in a shorter time.
Let all students present their values and write them on the board, grouping similar values from different student pairs. Together you calculate the average value of he electrical field for each grouping. Use that to calculate the electrical charge of the droplets. See table below for a brief example:
| E (measured) | E (average) | q=mg/E |
|---|---|---|
| 61200 | ||
| 61300 | ||
| 61200 | ||
| 61400 | ||
| 61600 | 61340 | 1.60·10-19 |
| 30300 | ||
| 30700 | ||
| 30500 | ||
| 30400 | 30475 | 3.22·10-19 |
| 20600 | ||
| 20200 | ||
| 20600 | ||
| 20500 | ||
| 20400 | 20425 | 4.80·10-19 |
References

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Contact :: Updated: 2019-01-01